Oxidative stress in plants
Ever since the introduction of molecular oxygen (O2)
into our atmosphere, by O2-evolving
photosynthetic organisms, about two billion years ago, reactive oxygen
species (ROS; also known as reactive oxygen intermediates, ROI) have
been the unwelcome companions of aerobic metabolism. In contrast to O2,
these partially reduced or activated derivatives of oxygen (O21,
O2-, H2O2,
and HO.) are highly reactive and toxic, and
can lead to the oxidative destruction of cells. Consequently, the
evolution of all aerobic organisms has been dependent upon the
development of efficient ROS-scavenging mechanisms. In recent years a
new role for ROS was identified: the control and regulation of
biological processes such as programmed cell death, hormonal signaling,
stress responses, and development. These studies extend our
understanding of ROS and suggest a dual role for ROS in plant biology: (a).
Toxic byproducts of aerobic metabolism, and (b).
Key regulators of metabolic and defense pathways.
The steady state level of ROS in the different cellular compartments is determined by interplay between multiple ROS-producing pathways, and ROS-scavenging mechanisms. These are controlled by the ROS-signal transduction pathway and constitute the “basic ROS cycle”. During normal growth and development this pathway monitors the level of ROS, produced by aerobic metabolism, and controls the expression and activity of ROS-scavenging pathways (Fig. 1). The basic ROS cycle may also perform fine metabolic tuning, e.g., suppression of photosynthesis, to reduce the production rate of ROS. There are many potential sources of ROS in plants (Table 1). Some are reactions of normal aerobic metabolism, such as photosynthesis and respiration, while others belong to pathways enhanced during abiotic stresses, such as photorespiration. In recent years new sources of ROS were identified in plants, including NADPH oxidases, amine oxidases, and cell wall-bound peroxidases. These are tightly regulated and participate in the control of processes such as programmed cell death, stress response, and pathogen defense.
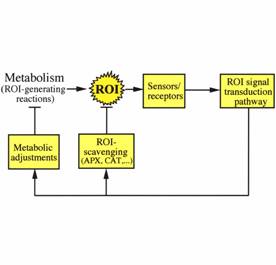 Fig.1.The
basic ROS cycle. This cycle modulates the cellular levels of ROS during
normal metabolism. Some of the key ROS scavenging enzymes of plants,
ascorbate peroxidase(APX), and catalase(CAT) are indicated.
Fig.1.The
basic ROS cycle. This cycle modulates the cellular levels of ROS during
normal metabolism. Some of the key ROS scavenging enzymes of plants,
ascorbate peroxidase(APX), and catalase(CAT) are indicated.ROI=ROS
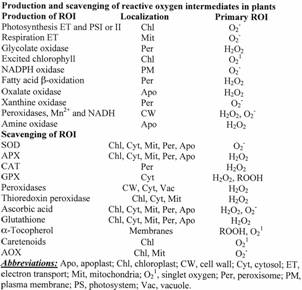 Table 1. Mechanisms for
production and scavenging of ROS in cells. AOX, alternative oxidase;
APX, ascorbate
peroxidase; CAT, catalase; GPX, glutathione;
peroxidase; SOD,
superoxide dismutase.
Table 1. Mechanisms for
production and scavenging of ROS in cells. AOX, alternative oxidase;
APX, ascorbate
peroxidase; CAT, catalase; GPX, glutathione;
peroxidase; SOD,
superoxide dismutase.
ROI=ROS
Under optimal growth conditions the production of ROS in cells is estimated at a constant rate of 240 µM s-1 O2-, and a steady state level of 0.5 µM H2O2. However, stresses that disrupt the cellular homeostasis of cells result in the enhanced production of ROS (up to 720 µM s-1 O2-, and a steady state level of 5-15 µM H2O2). These include drought and desiccation, salt, chilling, heat shock, heavy metals, UV radiation, air pollutants such as ozone and SO2, mechanical stress, nutrient deprivation, pathogen attack, and high light. The enhanced production of ROS during stress can pose a threat to cells, and many stress conditions enhance the expression of ROS-scavenging enzymes. However, it is also thought that during stress ROS are actively produced by cells (e.g., by NADPH oxidase), and act as signals for the induction of defense pathways. Thus, ROS may be viewed as cellular byproducts of stress metabolism, as well as secondary messengers involved in the stress-response signal transduction pathway. This view, of the “extended ROS cycle”, is presented in Fig. 2.
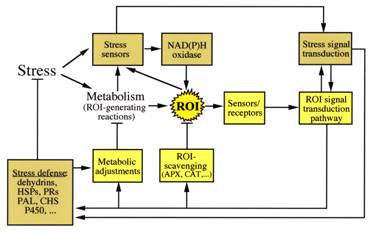
Fig. 2.The extended ROS cycle. This cycle operates in plants during biotic or abiotic stresses. HSPs, heat shock proteins; PR, pathogenesis related proteins; PAL, phenylalanine ammonia-lyaze; CHS, chalcone synthase; P450, cytochrome P450. ROI=ROS.
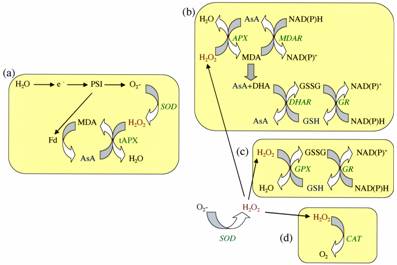
Fig. 3. The main cellular pathways for ROS removal in plants. (a). SOD and thylakoid-bound APX (tyl-APX) in the chloroplast as part of the water-water cycle. (b). SOD and APX in the stroma, cytosol, mitochondria, and apoplast of plants, as part of the ascorbate-glutathione pathway. (c). Glutathione peroxidase (GPX) and its glutathione regenerating cycle. (d). Catalase in peroxisomes. AsA, ascorbate; GSH and GSSG, reduced and oxidized glutathione; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; MDA, monodehydroascorbate; MDAR, monodehydroascorbate reductase; GR, glutathione reductase; PSI, photosystem I; Fd, ferredoxin; e-, electron.
Because ROS are toxic but also participate in key signaling events, plant cells require different mechanisms to regulate their intracellular ROS concentrations by scavenging of ROS. Major ROS scavenging mechanisms of plants are summarized in Table 1. These include superoxide dismutase (SOD; Fig. 3), ascorbate peroxidase (APX; Fig. 3), and catalase (CAT; Fig. 3). The balance between SOD, and APX (and/or CAT) activity in cells is considered to be crucial for determining the steady-state level of O2- and H2O2. This balance, together with sequestering of metal ions such as Fe and Cu by ferritin and copper-binding proteins, is thought to be important to prevent the formation of the highly toxic HO. via the metal-dependent Haber–Weiss or the Fenton reactions. Antioxidants such as ascorbic acid and glutathione, found at very high concentrations in chloroplasts and other cellular compartments (5-20 mM ascorbic acid and 1-5 mM glutathione), are also important for the defense of plants against oxidative stress. Consequently, mutants with suppressed ascorbic acid levels, and transgenic plants with suppressed ROS-scavenging enzymes, are hypersensitive to pathogen attack and abiotic stress conditions. In addition, over-expression of ROS-scavenging enzymes increases the tolerance of plants to abiotic stresses. ROS production can also be decreased in cells by the alternative channeling of electrons in the electron transport chains of the chloroplasts and mitochondria by a group of enzymes called alternative oxidases.
ROS at the interface between biotic and abiotic stresses
ROS play a central role in the defense of plants against pathogens. During this response, ROS are produced by plant cells via the enhanced enzymatic activity of plasma membrane-bound NADPH oxidases, cell wall-bound peroxidases and amine oxidases in the apoplast. H2O2 produced during this response is thought to diffuse into cells through aquaporins and together with salicylic acid (SA) and nitric oxide (NO) activate many of the plant defenses, including the induction of programmed cell death. The activity of APX and CAT is suppressed during this response by salicylic acid and nitric oxide, the expression of APX is post-transcriptionally suppressed, and the expression of CAT is down regulated at the steady-state mRNA level. Thus, the plant simultaneously produces more H2O2 and diminishes its own capability to scavenge H2O2, resulting in the over-accumulation of H2O2 and the activation of PCD. This response serves as an excellent example to how the steady state level of ROS can dramatically increase in cells when the basic ROS cycle is severed.
The ROS signal transduction pathway
Recent studies identified a number of components that may be involved in the ROS signal transduction of plants. These include the MAPKKK, AtANP1 (also NPK1), the MAPKs, AtMPK3/6, and Ntp46MAPK, and calmodulin. A hypothetical model depicting some of the players involved in this pathway is shown in Fig. 4. A sensor that might be a two component Histidine-kinase, or a receptor-like protein kinase, is thought to sense H2O2. Calmodulin and a MAPK cascade are then activated resulting in the induction/activation/suppression of a number of transcription factors. These regulate the response of plants to oxidative stress. Cross-talk with the pathogen-response signal transduction pathway (gene-for-gene) also occurs and may involve interactions between different MAPK pathways, feedback loops, and the action of salicylic acid and nitric oxide.

Fig. 4. A hypothetical model of the signaling pathway activated in plants in response to external application of oxidants. SA, salicylic acid; NO, nitric oxide; PCD, programmed cell death; HSP, heat shock protein; MAPK, mitogen-activated protein kinase; Tyr, tyrosine; HSF, heat shock transcription factor.
